Furosemide
Synonyms: furosemide, "Frusemide", "Lasix", "Furanthril", "Furosemid", "Errolon", "Fusid", "Frusemin", "Trofurit", "Rosemide", "Fuluvamide", "Desdemin", "Macasirool", "Fursemide", "Furantril", "Furanthryl", "Beronald", "Prefemin", "Lowpstron", "Aisemide", "Radonna", "Fulsix", "Transit", "Katlex", "Seguril", "Furesis", "Lasilix", "Lasex", "Salix", "Urex", "Frusetic", "Fursemid", "Eutensin", "Promedes", "Lazix", "Frusid", "Urosemide", "Profemin", "Furosedon", "Furanturil", "Frusemid", "Frusenex", "Rusyde", "Dryptal", "Yidoli", "Uremide", "Diural", "Impugan", "Uresix", "Aluzine", "Laxur", "Urian", "Disal", "Hydro-rapid", "Apo-Frusemide", "Synephron", "Spirofur", "Selectofur".
Source: Furosemide is a loop diuretic that is commonly used in the treatment of edematous states associated with cardiac, renal, hepatic failure and in the treatment of uncontrolled hypertension with abnormal renal function.
Identifiers:
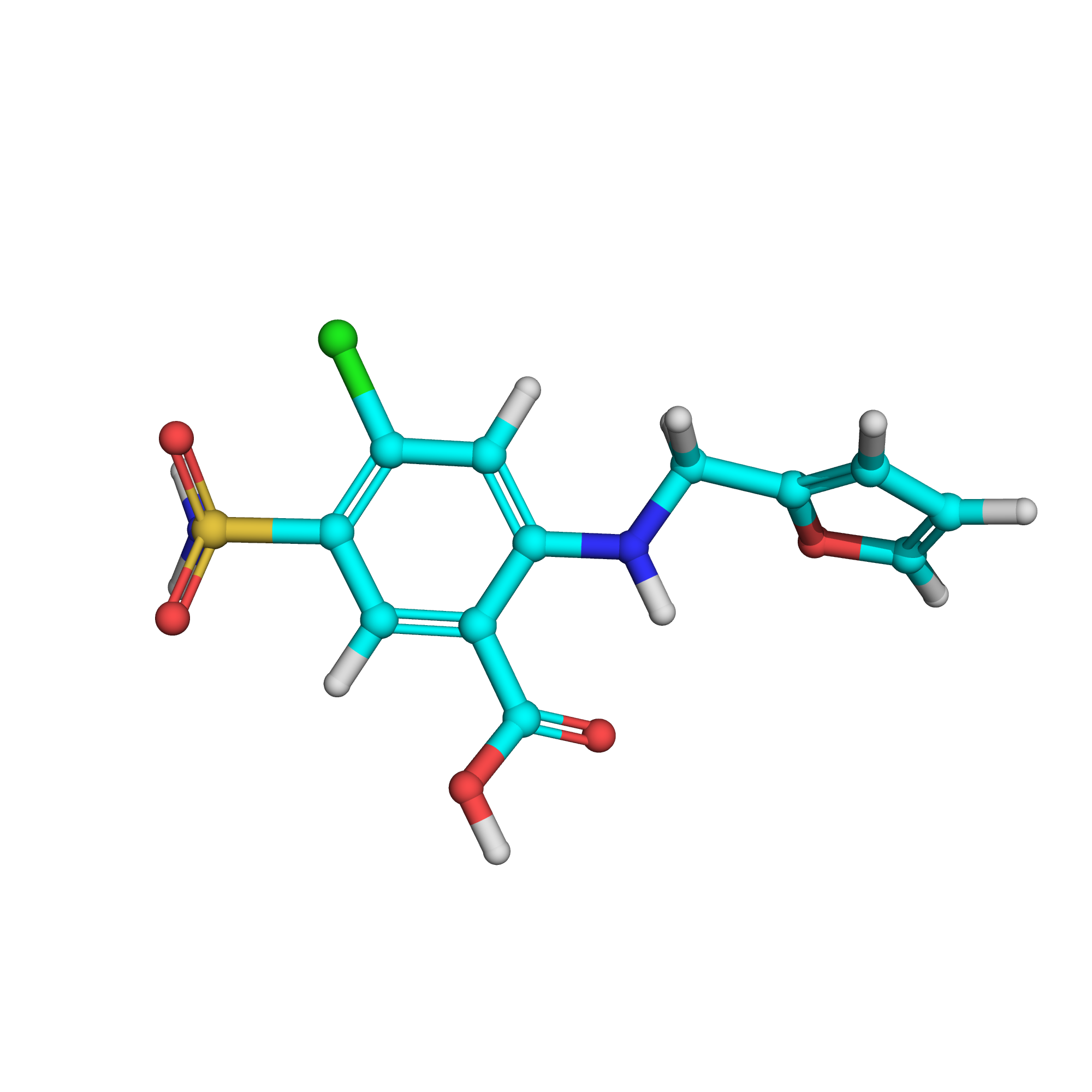
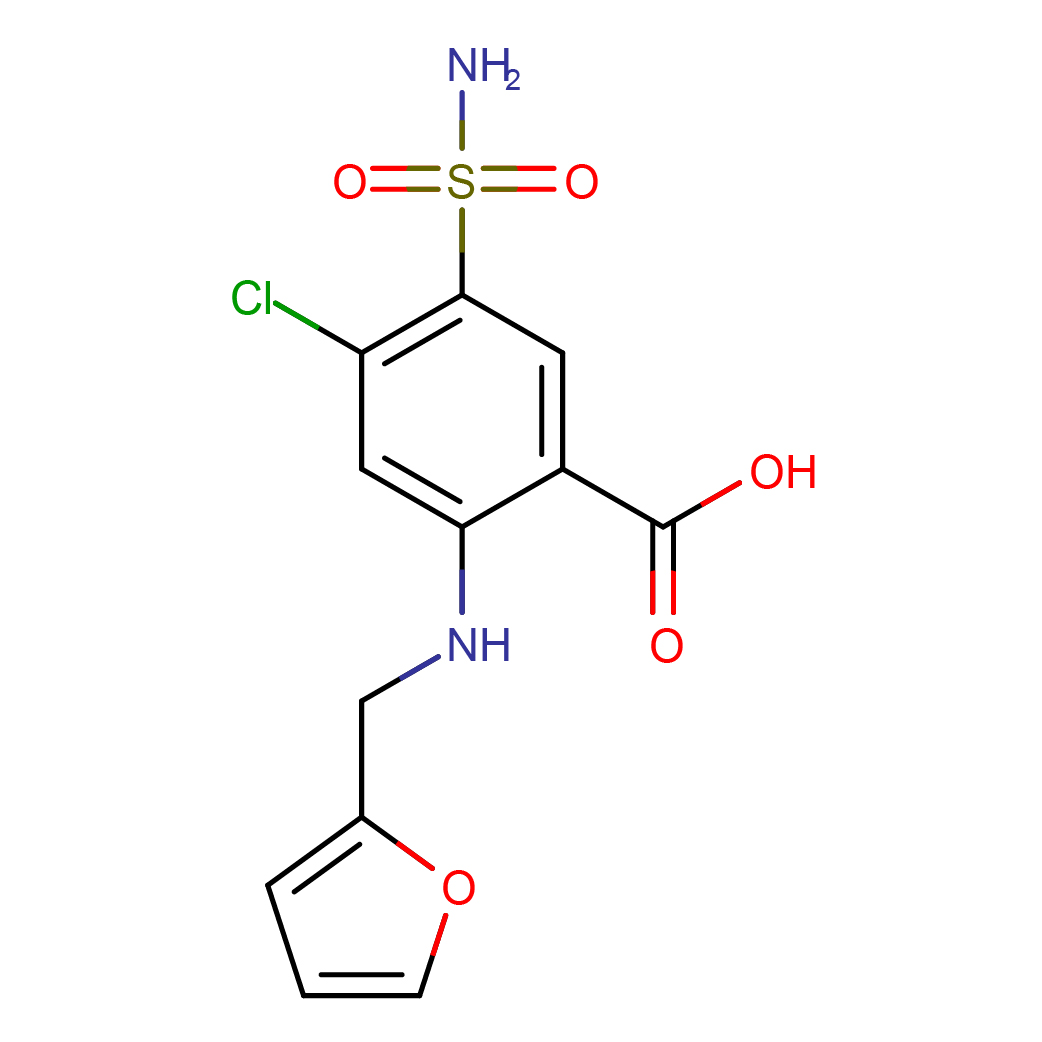
IUPAC Name: 4-chloro-2-(furan-2-ylmethylamino)-5-sulfamoylbenzoic acid
CAS Number: 54-31-9
PubChem ID: 3440
InChiKey: ZZUFCTLCJUWOSV-UHFFFAOYSA-N
Canonical SMILES: C1=COC(=C1)CNC2=CC(=C(C=C2C(=O)O)S(=O)(=O)N)Cl
Structural Properties:
Molecular Formula: C12H11ClN2O5S
Molecular Weight: 330.74
Pharmacophore Features:
Number of bond donors: 3
Number of bond acceptors: 7
Number of atoms different from hydrogen: 21
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Emerging Contaminant

Olvera?Vargas, H., Oturan, N., Buisson, D., Van Hullebusch, E. D., & Oturan, M. A. (2015). Electro?Oxidation of the Pharmaceutical Furosemide: Kinetics, Mechanism, and By?Products.?CLEAN?Soil, Air, Water,?43(11), 1455-1463.
External Links

2D-structure
3D-structure