Paroxetine
Synonyms: paroxetine,"Paxil", "Paxil CR", "Seroxat", "Paxetil", "Frosinor", "Paroxetinum", "Paroxetina", "Motivan", "Casbol", "Aropax", "PaxPar", "BRL 29060", "FG 7051", "Pexeva", "(3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine", "(3s,4r)-3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine", "[3H]Paroxetine", "Paroxetine Acetate", "(3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine"
Source: Paroxetine is used to treat depression, obsessive-compulsive
disorder, anxiety disorders and post-traumatic stress disorder
Identifiers:
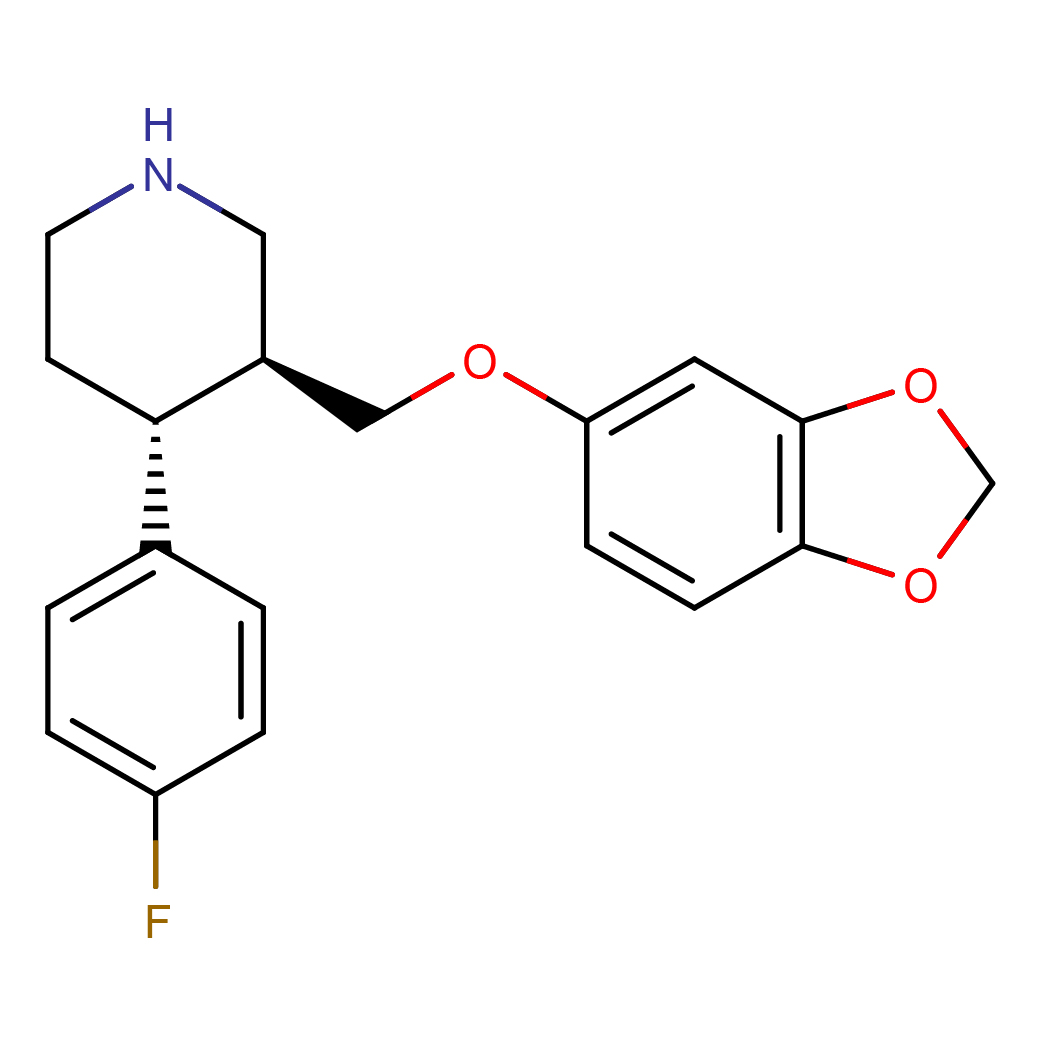
IUPAC Name: (3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine
CAS Number: 61869-08-7
PubChem ID: 43815
InChiKey: AHOUBRCZNHFOSL-YOEHRIQHSA-N
Canonical SMILES: C1CNCC(C1C2=CC=C(C=C2)F)COC3=CC4=C(C=C3)OCO4
Structural Properties:
Molecular Formula: C19H20FNO3
Molecular Weight: 329,400
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 5
Number of atoms different from hydrogen: 24
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Emerging Contaminant

Banjac, Z., Ginebreda, A., Kuzmanovic, M., Marc?, R., Nadal, M., Riera, J. M., & Barcel?, D. (2015). Emission factor estimation of ca. 160 emerging organic microcontaminants by inverse modeling in a Mediterranean river basin (Llobregat, NE Spain). Science of the Total Environment, 520, 241-252.
External Links

2D-structure
3D-structure