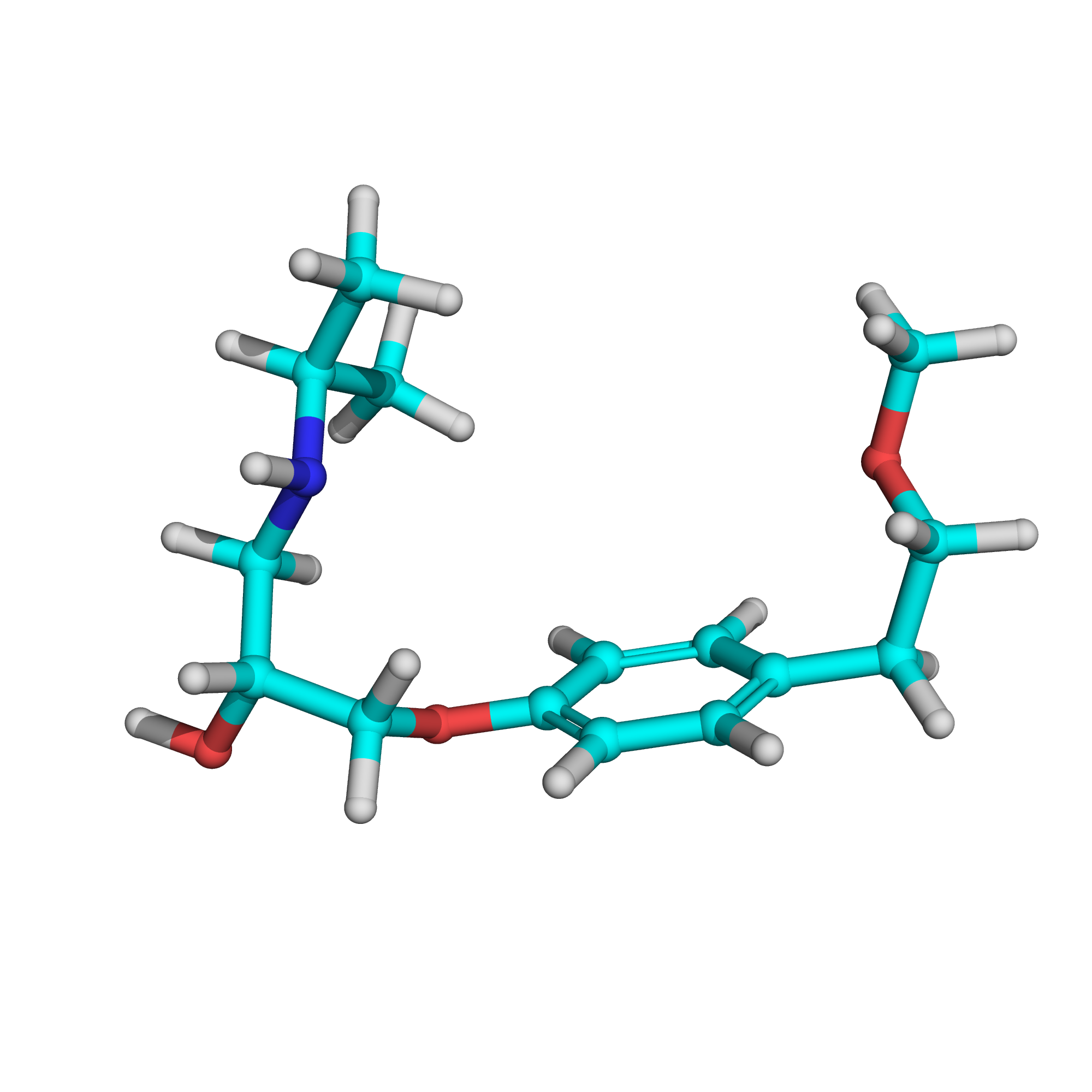
Metoprolol
Synonyms: metoprolol, Spesicor", "Beatrolol", "Toprol", "(RS)-Metoprolol", "dl-Metoprolol", "Lopressor", "37350-58-6", "Meijoprolol", "Metoprololum", "Preblok", "Presolol", "Metohexal", "Seroken", "Spesikor", "Lopresor", "Betalok", "Metoprolol succinate", "Selo-Zok", "Lopresoretic", "1-(Isopropylamino)-3-(4-(2-methoxyethyl)phenoxy)propan-2-ol", "1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol".
Source: Metoprolol is a selective ?1 (adrenergic) receptor blocker. Metoprolol has been used to treat various cardiovascular disorders including angina, arrhythmias, tachycardia, myocardial infarction, heart failure and hypertension.
Identifiers:
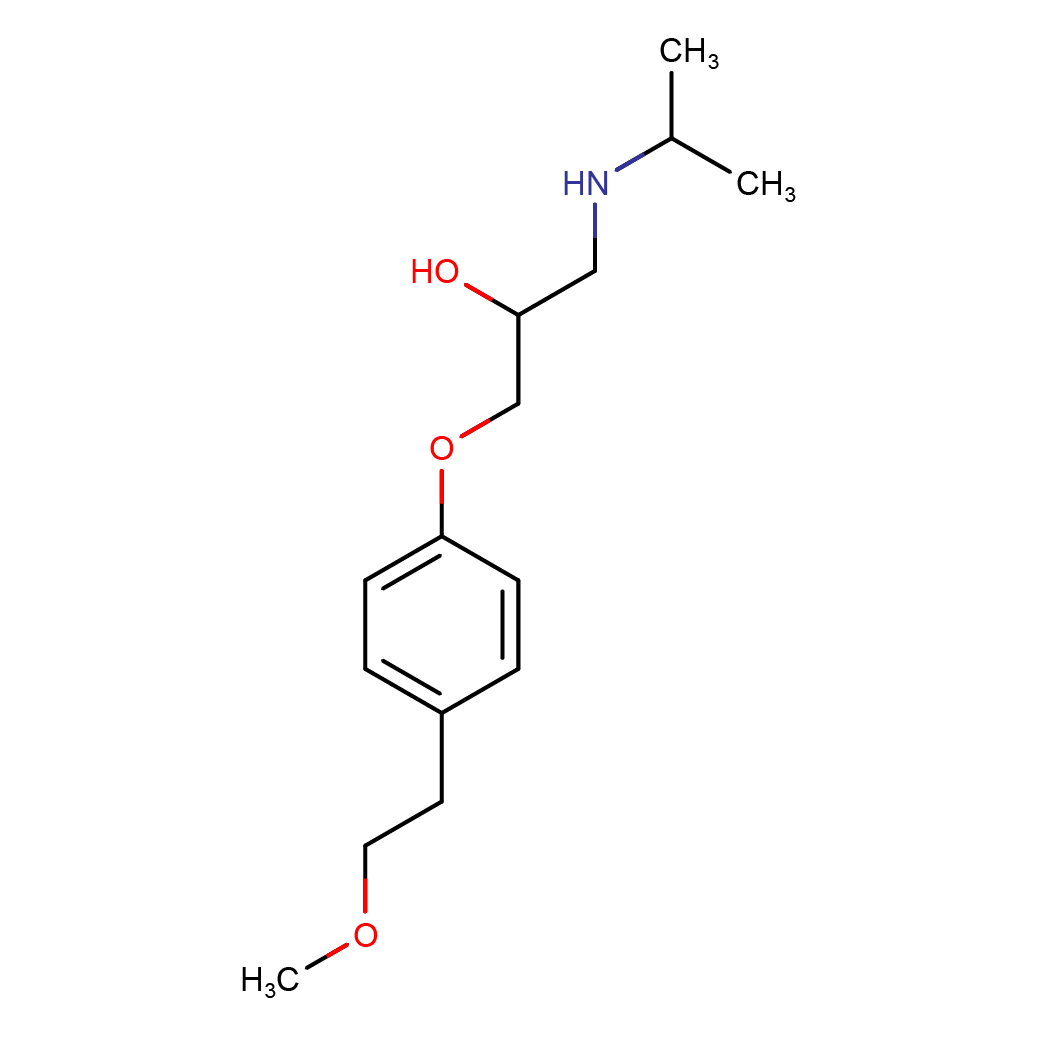
IUPAC Name: 1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol
CAS Number: 51384-51-1
PubChem ID: 4171
InChiKey: IUBSYMUCCVWXPE-UHFFFAOYSA-N
Canonical SMILES: CC(C)NCC(COC1=CC=C(C=C1)CCOC)O
Structural Properties:
Molecular Formula: C15H25NO3
Molecular Weight: 267.36
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 4
Number of atoms different from hydrogen: 19
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Emerging Contaminant

Lv, M., Lo, C., Hsu, C. C., Wang, Y., Chiang, Y. R., Sun, Q., ... & Yu, C. P. (2018). Identification of Enantiomeric Byproducts During Microalgae-Mediated Transformation of Metoprolol by MS/MS Spectrum Based Networking.?Frontiers in microbiology,?9, 2115.
Daneshkhah, M., Hossaini, H., & Malakootian, M. (2017). Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. Journal of environmental chemical engineering, 5(4), 3490-3499.
External Links

2D-structure
3D-structure