Coprosterol
Synonyms: Coprosterol, "Stercorin", "5beta-Cholestan-3beta-ol", "Koprosterol", "Koprosterin", "5beta Coprostanol", "5-beta-Cholestan-3-beta-ol", "3-beta-Cholestanol", "Coprostan-3-beta-ol", "3-beta-Hydroxycholestane", "Cholestan-3-ol,5.beta.)-", "3beta-Hydroxy-5beta-cholestanol", "5.beta.-Cholestan-3.beta.-ol", "Coprostan-3-ol", "(3-beta,5-beta)-Cholestan-3-ol"
Source: Coprosterol is formed by microbiological reduction of cholesterol in the lower intestinal tract.
Identifiers:
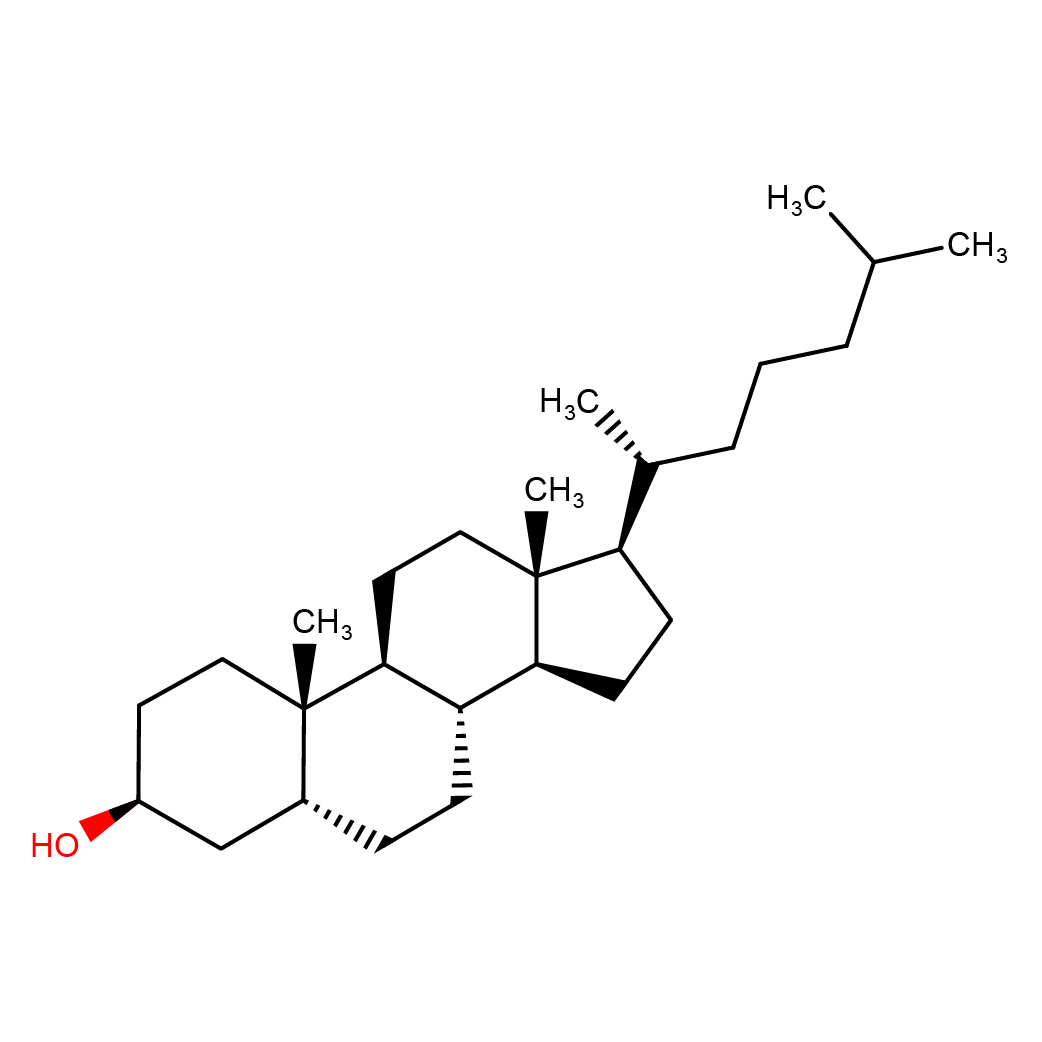
IUPAC Name: (3S,5R,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
CAS Number: 360-68-9
PubChem ID: 221122
InChiKey: QYIXCDOBOSTCEI-NWKZBHTNSA-N
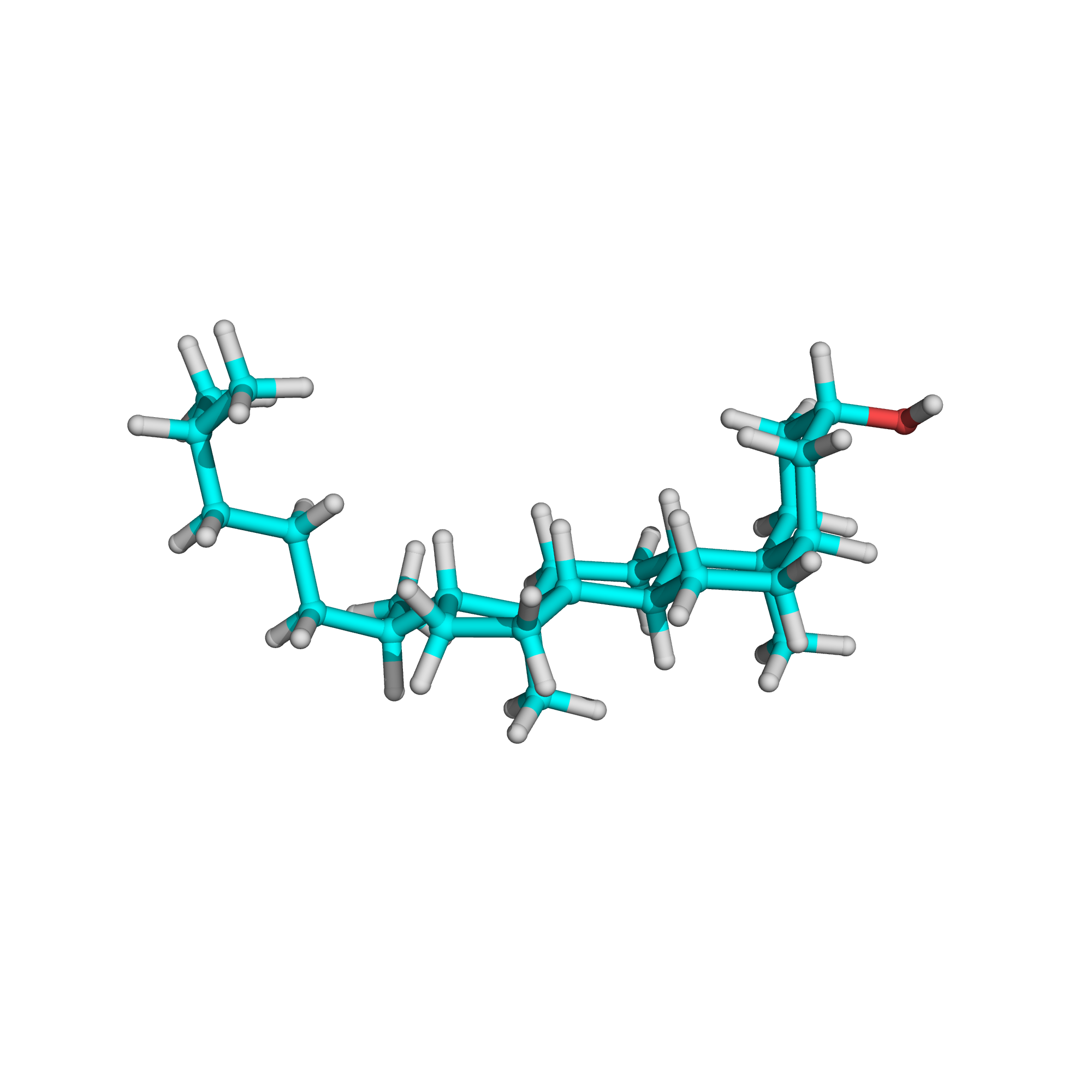
Canonical SMILES: CC(C)CCCC(C)C1CCC2C1(CCC3C2CCC4C3(CCC(C4)O)C)C
Structural Properties:
Molecular Formula: C27H48O
Molecular Weight: 388,700
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 28
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Emerging Contaminant

Baldwin, A. K., Corsi, S. R., De Cicco, L. A., Lenaker, P. L., Lutz, M. A., Sullivan, D. J., & Richards, K. D. (2016). Organic contaminants in Great Lakes tributaries: Prevalence and potential aquatic toxicity. Science of the Total Environment, 554, 42-52.
External Links

2D-structure
3D-structure